THERMOGRAPHY-CONTROLLED
wIRA SUPERFICIAL HYPERTHERMIA
EFFECTIVE RADIOSENSITIZER FOR LOW-DOSE (RE)IRRADIATION
CLINICAL EFFECTS IN LOCALLY
RECURRENT BREAST CANCER
Local recurrence of breast cancer is frequently a therapeutic challenge.
In case of irresectability and pre-irradiation, effective tumor control by re-irradiation faces the risk of cumulative radiotoxicity. These heavily pretreated patients are often also resistant to systemic therapies, or the side-effects of these therapies must be weighed against the limited anti-tumor effects that can be expected.
Overall response rate in 201 patients was 95% (see the detailed response and control rates for different size classes in Notter et al 2020).
In case of resectability, R1 resection (“microscopic disease”) or low margins cause a high risk of local re-recurrence. The same schedule of combined HT/RT can be used as adjuvant re-RT.
Of course, wIRA superficial hyperthermia can also be combined with other RT schedules, such as the “Dutch schedule” (total re-RT dose 32 Gy) or other standard radiation protocols.
HYPERTHERMIA AS A RADIO-
AND CHEMOSENSITIZER
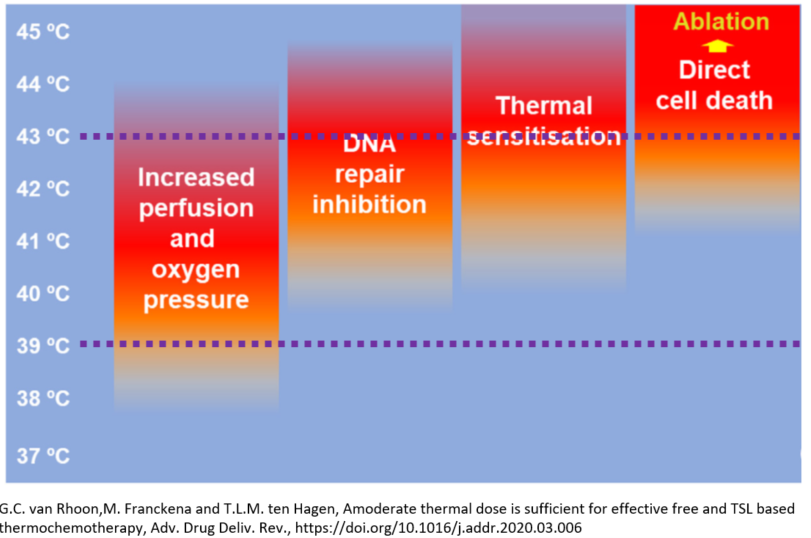
Sensitizing mechanisms:
- increasing tumor perfusion and oxygenation
- inhibiting DNA repair
- increasing antitumor immune activity
While different temperatures are optimal for these mechanisms, the whole temperature range of 39-43°C has been described as effective in combined hyperthermia/radiotherapy (HT/RT).
In order to achieve the most beneficial effects, the time gap between hyperthermia and radiotherapy treatment should be as short as possible.
Using thermography-controlled wIRA superificial hyperthermia, (re-)irradiation is performed few minutes after completion of a 45-60 min hyperthermia session.
This combination and timing enables effective tumor control with
- significantly reduced RT dosage and toxicity
- option of safe re-irradiation even in cases where re-irradiation in therapeutic dosage alone is not an option or associated with the risk of severe side-effects.
TECHNIQUE
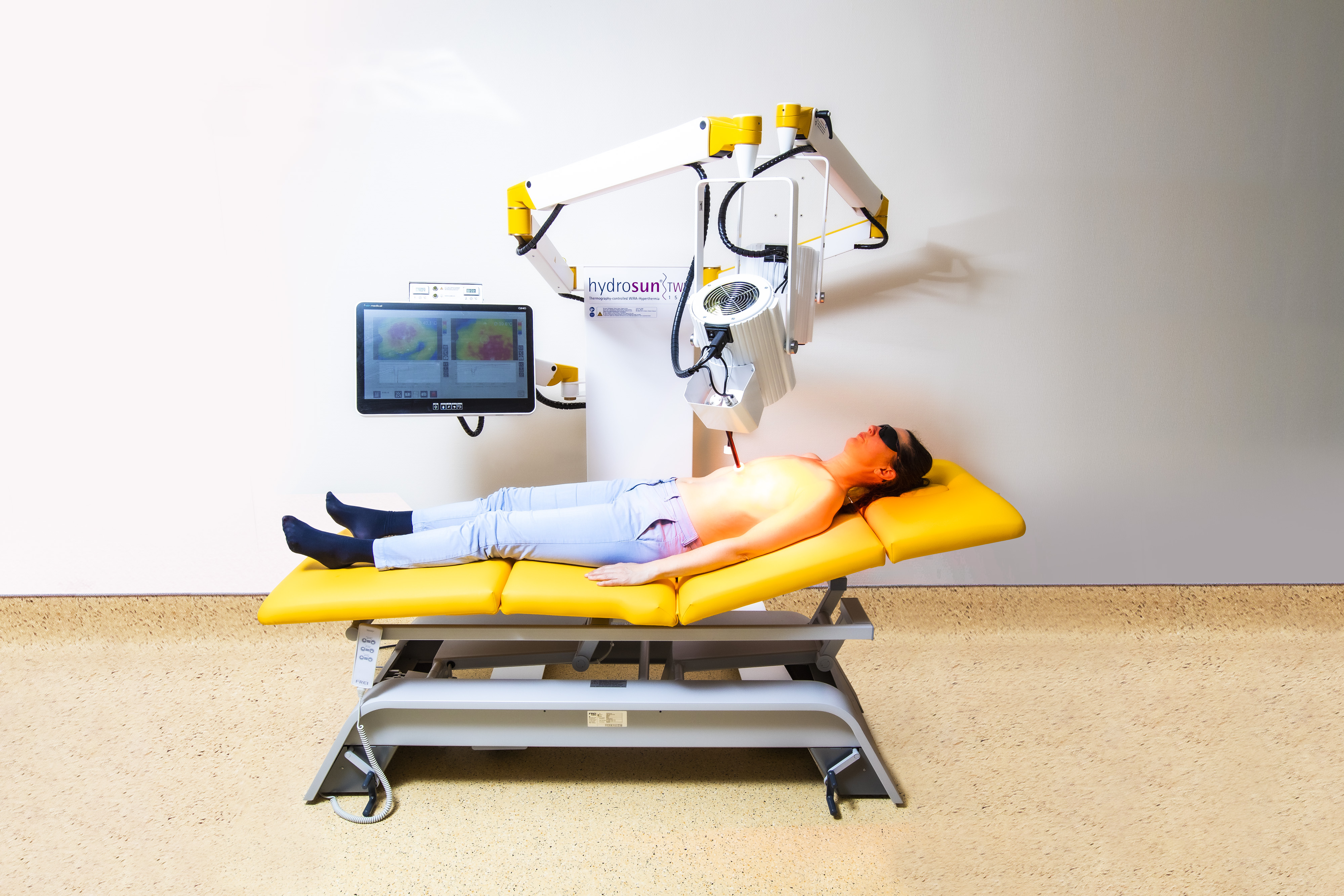
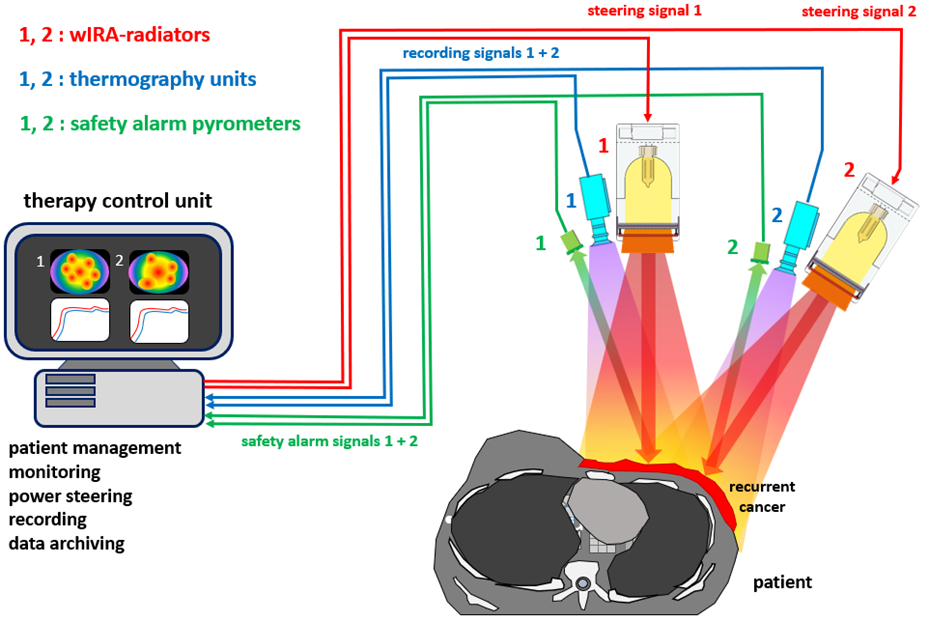
The hydrosun®TWH1500, using water-filtered infrared A with a high and most compliant energy input to deeper tissue layers is the first oncological local hyperthermia device that enables a contact-free supply of energy.
This allows for continous real-time thermographic control with automatic switch on and off control functions making optimal and safe energy input possible, while achieving an effective heating depth of at least 15mm (>40°C) respectively 20mm (>39.5°C) if maximum skin temperature is set at 42- 43°C.
Thus, the incidence of thermal skin damage (TSD) ≥ grade 2 has been reduced to < 1 %. Additionally, the physiological reactions in the treatment area can be observed real-time and analyzed even after the session has ended.
The treatment is most agreeable for the patient.
The patient does not suffer from any body fixation or painful applicator contact, e.g. with ulcerated lesions.
By using a twin-applicator system, the performance of the hydrosun®TWH1500 is unaffected by heterogenous body contours and it easily covers large treatment areas for effective hyperthermia of large-sized chest wall recurrence, especially in lymphangiosis carcinomatosa.
Thermography-controlled wIRA superficial hyperthermia is cited as an approved method in the "Quality assurance guidelines for superficial hyperthermia clinical trials", issued by ESHO (European Society for Hyperthermic Oncology) and published in Int J Hyperthermia. 2017 and Strahlenther Onkol 2017.
wIRA SUPERFICIAL HYPERTHERMIA BASICS AND CLINICAL RESULTS
Download the Key Publications:
Notter M, Piazena H, Vaupel P:
Hypofractionated re-irradiation of large-sized re-current breast cancer with thermography-controlled, contact-free water-filtered infrared-A hyperthermia: A retrospective study of 73 patients.
International Journal of Hyperthermia Volume 33, 2017 - Issue 2, pp. 227-236
Vaupel P, Piazena H, Müller W, Notter M:
Biophysical and photobiological basics of water-filtered infrared-A hyperthermia of superficial tumors.
Int J Hyperthermia, Volume 35, 2018 - Issue 1, pp. 26–36
Notter M, Thomsen AR, Nitsche M, Hermann RM, Wolff H, Habl G, Münch K, Grosu AL, Vaupel P:
Combined wIRA-Hyperthermia and Hypofractionated Re-Irradiation in the Treatment of Locally Recurrent Breast Cancer: Evaluation of Therapeutic Outcome Based on a Novel Size Classification.
Cancers 2020, 12, 606; doi:10.3390/cancers12030606
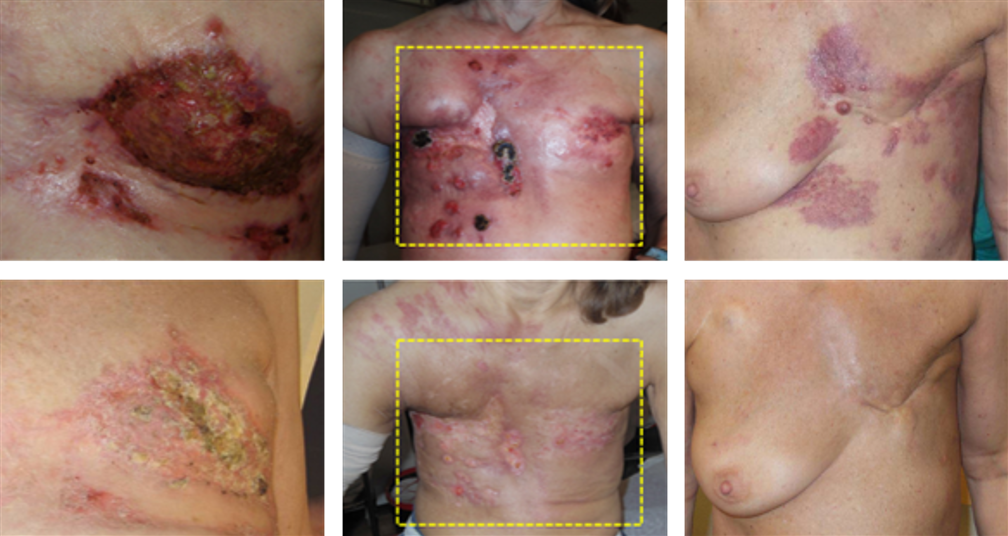
Notter M, Stutz E, Thomsen AR, Vaupel P:
Radiation-Associated Angiosarcoma of the Breast and Chest Wall Treated with Thermography-Controlled, Contactless wIRA-Hyperthermia and Hypofractionated Re-Irradiation.
Cancers 2021, 13(15), 3911; doi: 10.3390/cancers13153911

Referring to a review of Buchholz et al on Multidisciplinary Management of Locoregional Recurrent Breast Cancer in the Journal of Clinical Oncology, a correspondence of Thomsen AR et al (University Medical Center Freiburg) has been published "open access", along with a confirming and most positive reply from Buchholz TA, Ali S, Hunt KK:
Thomsen AR, Vaupel P, Grosu AL, Notter M:
Hyperthermia Plus Re-Irradiation in the Management of Unresectable Locoregional Recurrence of Breast Cancer in Previously Irradiated Sites
Buchholz TA, Ali S, Hunt KK:
Reply to A. Thomsen et al.
"Considering this, we commend Notter et al. for publishing their experience of treating 201 patients with locally recurrent breast cancer by using a modern hyperthermia technology that allowed for large-field hyperthermia combined with low-dose re-irradiation. They report excellent rates of tumor response, local control, and locally progressive free survival. We agree with Notter et al. that a confirmatory phase III comparative trial is not feasible, and therefore we find their recent publication to add to options for therapeutic consideration."
REFERENCES
SWITZERLAND:
- Lindenhofspital Bern, Dept. of Radiation Oncology
- Kantonsspital Aarau, Department of Radiation Oncology
- Kantonsspital Winterthur, Department of Radiation Oncology
- Kantonsspital Luzern, Department of Radiation Oncology
- ZIO in der Radiotherapie Hirslanden Männedorf bei Zürich
NETHERLANDS:
- Amsterdam UMC, Dept. of Radiation Oncology
UK:
- Guy's Cancer Center / King's College London
GERMANY:
- University Medical Center Freiburg, Dept. of Radiation Oncology
- Radioonkologie und Strahlentherapie Bremen
- Radiologie München, Strahlentherapie
- University Magdeburg, Medical Center, Dept. of Radiation Oncology
- Heidelberg University Hospital, Department of Radiation Oncology
- Klinikum Stuttgart, Stuttgart Cancer Center (SCC)
AUSTRIA:
- Ordensklinikum Linz Barmherzige Schwestern, Dept. of Radiation Oncology
- Strahlenklinik der Medizinischen Universität Graz
PORTUGAL:
WEBINAR